Abdominal Abscess
Abdominal abscess continues to be an important and serious problem in surgical practice. Appropriate treatment is often delayed because of the obscure nature of many conditions, resulting in abscess formation and making diagnosis and localization difficult. Associated pathophysiologic effects may become life-threatening or lead to extended periods of morbidity with prolonged hospitalization. Delayed diagnosis and treatment can also lead to increased mortality; therefore, the economic impact of delaying treatment is significant.
A better understanding of intra-abdominal abscess pathophysiology and a high clinical index of suspicion should allow earlier recognition, definitive treatment, and reduced morbidity and mortality. [1]
Treatment involves antibiotic therapy and abscess drainage (percutaneous, open, or laparoscopic).
Pathophysiology
Intra-abdominal abscesses are localized pus collections confined in the peritoneal cavity by an inflammatory barrier. This barrier may include the omentum, inflammatory adhesions, or contiguous viscera. The abscesses usually contain a mixture of aerobic and anaerobic bacteria from the gastrointestinal (GI) tract.
Bacteria in the peritoneal cavity, particularly those arising from the large intestine, stimulate an influx of acute inflammatory cells. The omentum and viscera tend to localize the site of infection, producing a phlegmon. The resulting hypoxia in the area facilitates anaerobes’ growth and impairs granulocyte’s bactericidal activity. The phagocytic activity of these cells degrades cellular and bacterial debris, creating a hypertonic milieu that expands and enlarges the abscess cavity in response to osmotic forces.
If untreated, the process continues until bacteremia develops, which then progresses to generalized sepsis with shock.
Abdominal Abscess – Etiology
Although multiple causes of intra-abdominal abscesses exist, the following are the most common:
- Perforation of viscus, which includes peptic ulcer perforation [5]
- Perforated appendicitis and diverticulitis
- Gangrenous cholecystitis
- Mesenteric ischemia with bowel infarction
- Pancreatitis or pancreatic necrosis progressing to pancreatic abscess [6]
Other causes include untreated penetrating trauma to the abdominal viscera and postoperative complications, such as anastomotic leakage [1, 7] or missed gallstones during laparoscopic cholecystectomy.
Microbiology includes a mixture of aerobic and anaerobic organisms. The most commonly isolated aerobic organism is Escherichia coli, and the most commonly observed anaerobic organism is Bacteroides fragilis. A synergistic relationship exists between these organisms. In patients who receive prolonged antibiotic therapy, yeast colonies (eg, candidal species) or a variety of nosocomial pathogens may be recovered from abscess fluids.
Skin flora may be responsible for abscesses after a penetrating abdominal injury. Neisseria gonorrhoeae and chlamydial species are the most common organisms involved in pelvic abscesses in females as part of pelvic inflammatory disease. The type and density of aerobic and anaerobic bacteria isolated from intra-abdominal abscesses depend upon the nature of the microflora associated with the diseased or injured organ.
Microbial flora of the GI tract shifts from small numbers of aerobic streptococci, including enterococci and facultative gram-negative bacilli in the stomach and proximal small bowel, to larger numbers of these species, with an excess of anaerobic gram-negative bacilli (particularly Bacteroides species) and anaerobic gram-positive flora (streptococci and clostridia) in the terminal ileum and colon.
Differences in microorganisms observed from the upper portion of the GI tract to the lower portion partially account for differences in septic complications associated with injuries or diseases to the upper and lower gut. Sepsis occurring after upper GI perforations or leaks causes less morbidity and mortality than sepsis after leaks from colonic insults.
Abdominal Abscess – Prognosis
The introduction of computed tomography (CT) for the diagnosis and drainage of intra-abdominal abscesses has led to a dramatic reduction in mortality. Sequential, multiple organ failure is the main cause of death. Incidence of death is correlated to the severity of the underlying cause, a delayed diagnosis, inadequate drainage, and unsuspected foci of infection in the peritoneal cavity or elsewhere.
Risk factors for morbidity and mortality include the following :
- Multiple surgical procedures
- Age older than 50 years
- Multiple organ failure
- Complex, recurrent, or persistent abscesses
History and Physical Examination
Intra-abdominal abscesses are highly variable in presentation. Persistent abdominal pain, focal tenderness, spiking fever, persistent tachycardia, prolonged ileus, leukocytosis, or intermittent polymicrobial bacteremia suggest an intra-abdominal abscess in patients with predisposing primary intra-abdominal disease or in individuals who have had abdominal surgery. If a deeply seated abscess is present, many of these classic features may be absent. The only initial clues may be persistent fever, mild liver dysfunction, persistent gastrointestinal (GI) dysfunction, or nonlocalizing debilitating illness.
The diagnosis of an intra-abdominal abscess in the postoperative period may be difficult because postoperative analgesics and incisional pain frequently mask abdominal findings. In addition, antibiotic administration may mask abdominal tenderness, fever, and leukocytosis.
In patients with subphrenic abscesses, irritation of contiguous structures may produce shoulder pain, hiccups, or unexplained pulmonary manifestations, such as pleural effusion, basal atelectasis, or pneumonia. With pelvic abscesses, frequent urination, diarrhea, or tenesmus may occur. A diverticular abscess may present as an incarcerated inguinal hernia. [10]
Many patients have a significant septic response, suffer volume depletion, and develop a catabolic state. This syndrome may include high cardiac output, tachycardia, low urine output, and low peripheral oxygen extraction. Initially, respiratory alkalosis due to hyperventilation may occur. If left untreated, this progresses to metabolic acidosis. Sequential multiple organ failure is highly suggestive of intra-abdominal sepsis.
Differential Diagnoses
- Inflammatory Bowel Disease
- Persistent tachycardia
- Prolonged ileus
- Unexplained postoperative fever
Abdominal Abscess – Laboratory Studies
Appropriate hematologic studies should be done. Hematologic parameters suggestive of infection (eg, leukocytosis, anemia, abnormal platelet counts, and abnormal liver function) frequently are present, although patients who are debilitated or elderly often fail to mount reactive leukocytosis or fever.
Blood cultures indicating persistent polymicrobial bacteremia strongly implicate the presence of an intra-abdominal abscess. Because more than 90% of intra-abdominal abscesses contain anaerobic organisms, particularly B fragilis, postoperative Bacteroides bacteremia suggests intra-abdominal sepsis.
Radiography
Plain abdominal radiographs, though rarely diagnostic, frequently indicate the need for further investigation. [11] Abnormalities on plain abdominal films may include a localized ileus, extraluminal gas, air-fluid levels, mottled soft-tissue masses, absence of psoas outlines, or displacement of viscera.
In subphrenic or even subhepatic abscesses, the chest radiograph may show pleural effusion, elevated hemidiaphragm, basilar infiltrates, or atelectasis.
Ultrasonography
Ultrasonography (US) is readily available, portable, and inexpensive. The findings can be quite specific when correlated with the clinical picture. In experienced hands, the US has an accuracy rate greater than 90% for diagnosing intra-abdominal abscesses. Bedside US is particularly useful for immobile, critically ill intensive care unit (ICU) patients.
A drawback of the US is that marked obesity, bowel gas, intervening viscera, surgical dressings, open wounds, and stomas can create problems with definition. In addition, the quality of the procedure is operator-dependent. These disadvantages may limit the efficacy of this modality in postoperative patients.
Computed Tomography
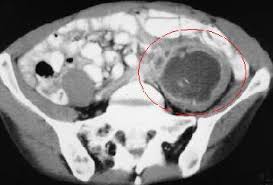
Computed tomography (CT) has greater than 95% accuracy and is the best diagnostic imaging method for abdominal abscess. The presence of ileus, dressings, drains, or stomas does not interfere with reliability.
For good anatomic resolution, use oral and intravenous (IV) contrast (see the images below). Oral contrast may help to differentiate a fluid-filled extraluminal structure from a normal intestine. Extravasation of oral contrast indicates a fistula or an anastomotic leak. IV contrast may enhance the abscess by concentrating the contrast material within the abscess wall. The use of oral and IV contrast may be limited by ileus, allergy to contrast material, and renal insufficiency.
Radioisotope Scanning
Typically, radioisotope scans provide no pertinent information that is not found with CT. The disadvantages of these scans limit their use to cases in which intra-abdominal abscesses are strongly suspected in a patient but US or CT has failed to provide adequate diagnostic information.
Pharmacologic Therapy
Pharmacologic therapy involves the empiric administration of parenteral empiric antibiotics. This should be initiated before abscess drainage and concluded when all systemic signs of sepsis have resolved. Because abscess fluid usually contains a mixture of aerobic and anaerobic organisms, initial empiric therapy must be directed against both types of microbes. This may be accomplished with antibiotic combination therapy or with broad-spectrum single-agent therapy. Specific therapy is then guided by the results of cultures retrieved from the abscess. [8]
In patients who are immunosuppressed, candidal species may play an important pathogenic role, and treatment with amphotericin B may be indicated.
Percutaneous Abscess Drainage
Drainage of pus is mandatory and is the first line of defense against progressive sepsis. Percutaneous computed tomography (CT)-guided catheter drainage has become the standard treatment for most intra-abdominal abscesses (see the image below).
It avoids anesthesia and possibly difficult laparotomy, prevents the possibility of wound complications from open surgery, and may reduce the length of hospitalization. It also obviates the possibility of contaminating other areas within the peritoneal cavity.
Percutaneous drainage, when feasible, is typically preferred to open drainage.
After drainage, clinical improvement should occur within 48-72 hours. Lack of improvement within this time frame mandates repeat CT to check for additional abscesses.
Surgical drainage becomes mandatory if residual fluid cannot be evacuated with catheter irrigation, manipulation, or additional drain placement.
Percutaneous drainage is effective in 90% of patients who have a single unilocular abscess with no enteral communication.
Complex abscesses that include multiple locations, interloop abscesses, or those associated with an enteric fistula may necessitate surgery. Surgical intervention also may be indicated for abscesses with tenacious contents, such as infected hematoma, infected pancreatic necrosis, or fungal abscesses.
Laparoscopic or Open Abscess Drainage
If percutaneous drainage fails or if collections are not amenable to catheter drainage, surgical drainage is an option.
The surgical approach may be either laparoscopic or open (laparotomic).
Laparoscopic drainage for a massive intra-abdominal abscess is minimally invasive, permitting exploration of the abdominal cavity without the use of a wide incision; purulent exudate can be aspirated under direct vision.
With accurate preoperative localization, direct open surgical drainage may be possible through an extraperitoneal open approach. This technique reduces the risk of bowel injury, contamination spread, and bleeding. It also allows for a faster return of bowel function.
The transperitoneal open approach is made safer by the judicious use of preoperative antibiotics. Although contamination of otherwise uninfected sites remains a major concern, this complication is particularly reduced if the organisms involved are sensitive to the chosen drugs. Transabdominal exploration of the entire peritoneal cavity allows fibrin debridement. It also permits complete bowel mobilization to locate and drain all synchronous abscesses, which occur in as many as 23% of patients.
Transperitoneal exploration is indicated for multiple abscesses not amenable to CT-guided drainage, such as interloop collections or an enteric fistula feeding the abscess. In the latter situation, draining the abscesses with an enteric communication may be possible for several days before a laparotomy is performed to control the fistula. This may allow some resolution of the inflammatory process, thus making surgery less difficult.
Pelvic abscesses often are palpable as tender, fluctuant masses impinging on the vagina or rectum. Draining these abscesses transvaginally or transrectally is best to avoid the transabdominal approach.
Improved clinical findings within 3 days after treatment indicate successful drainage. Failure to improve may indicate inadequate drainage or another source of sepsis. If left untreated, the septic state inevitably produces multiple organ failure.
The transabdominal open approach to intra-abdominal abscesses can be exceedingly difficult. Matted bowel, adhesions, and loss of anatomic integrity can pose severe problems. This is especially true when susceptible viscera, such as a loop of small bowel, intermittently adhere to the abscess wall or cavity. Therefore, whenever possible, CT-guided drainage is a valuable initial step.